- 2025-11-27
- 735
- 5 Comments
Precision Mold Parts: The Invisible Guardian of Safe & Reliable Medical Device Manufacturing
Precision Mold Parts: The Invisible Guardian of Safe & Reliable Medical Device Manufacturing
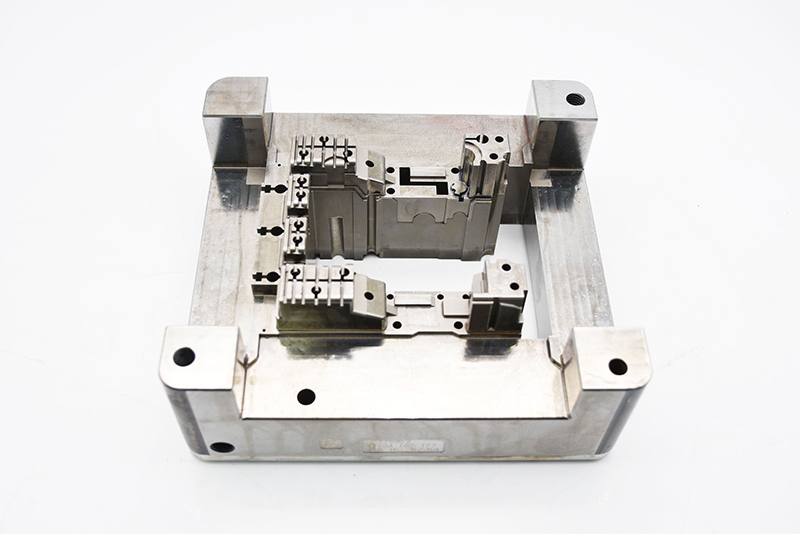
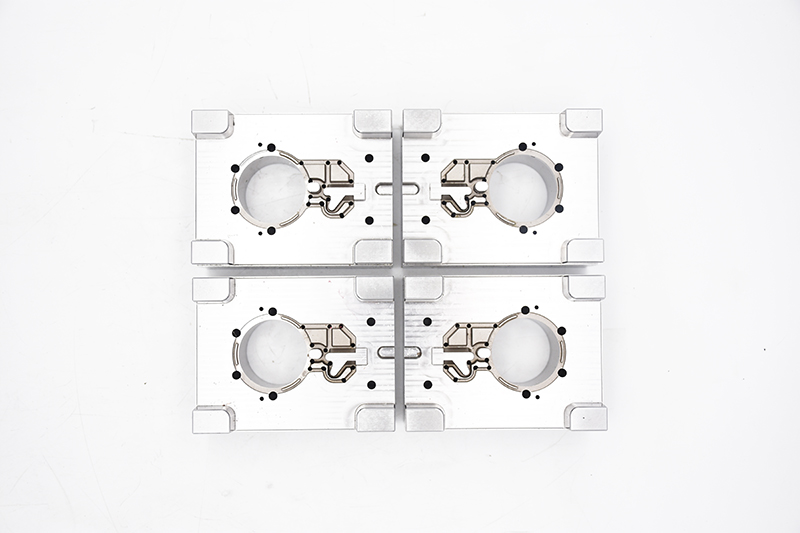
In the medical device industry—where every micron matters and safety is non-negotiable—there’s no room for error. From life-saving implantable devices to high-precision diagnostic equipment, the quality of medical products directly impacts patient health and clinical outcomes. Behind these critical devices lies a set of unsung components that define their precision, sterility, and durability: precision mold parts. Ejector pins, guide pins, bushings, cooling inserts, and valve gates may seem small, but they are the backbone of medical device manufacturing—ensuring that every component meets the strictest standards of accuracy, biocompatibility, and regulatory compliance. For medical device manufacturers striving to deliver safe, effective products, choosing the right precision mold parts isn’t just a supply chain decision—it’s a commitment to patient trust and clinical excellence.
Why Medical Device Manufacturing Demands Unmatched Precision in Mold Parts
Medical device manufacturing is one of the most regulated and demanding sectors globally, with requirements that far exceed general industrial standards. Unlike consumer goods or automotive components, medical devices must withstand rigorous sterilization, maintain dimensional stability in clinical use, and avoid any risk of contamination or material leaching. This places extraordinary demands on precision mold parts:
Micron-Level Accuracy for Critical Functionality: Implantable devices (e.g., pacemaker leads, orthopedic screws) and diagnostic components (e.g., lab-on-a-chip devices) require dimensional tolerances as tight as ±0.0005mm. A tiny deviation in a mold’s guide pin or ejector pin can lead to component misalignment, rendering a device ineffective or even dangerous for patients.
Sterility & Biocompatibility: Medical mold parts must be manufactured from materials that resist corrosion, avoid particle shedding, and withstand repeated sterilization (autoclaving, ethylene oxide, gamma radiation). Any contamination from mold parts—such as metal flakes or chemical residues—could compromise patient safety.
Regulatory Compliance: Medical device manufacturers must adhere to strict global standards (ISO 13485, FDA QSR 820, EU MDR) that require full traceability of all components, including mold parts. Precision mold parts must come with detailed material certificates, manufacturing records, and quality reports to support regulatory audits.
Consistency Across High-Volume Production: Diagnostic tools (e.g., syringes, test tubes) and disposable devices are produced in millions of units annually. Precision mold parts must maintain identical performance across 1 million+ cycles to ensure every device meets the same quality standards.
Key Medical Device Applications: How Precision Mold Parts Ensure Safety & Performance
Precision mold parts are integral to manufacturing a wide range of medical devices, from implantables to disposables. Below are the critical applications where these parts directly impact device safety, functionality, and compliance:
1. Implantable Medical Devices: Precision That Saves Lives
Implantable devices—such as cardiac stents, orthopedic hip implants, and neural stimulation leads—are placed inside the human body for years, requiring uncompromising precision and biocompatibility. Precision mold parts play a pivotal role in their production:
Micro-Mold Inserts for Stent Frame Manufacturing: Stents require ultra-thin, complex geometries to expand inside blood vessels. Our precision micro-mold inserts (machined with wire EDM technology) create stent frames with tolerances of ±0.001mm, ensuring uniform strut thickness and flexibility—critical for avoiding vessel damage during implantation.
Biocompatible Ejector Pins for Implant Housings: Implant housings (e.g., pacemaker casings) are made from medical-grade titanium or PEEK. Our ejector pins are coated with PTFE or ceramic to prevent metal-to-metal contact, eliminating particle shedding and ensuring the housing remains sterile and biocompatible. For a leading cardiac device manufacturer, our ejector pins reduced implant housing defects by 75% and eliminated post-implantation inflammation risks.
2. Diagnostic Equipment: Accuracy for Reliable Test Results
Diagnostic devices—such as in-vitro diagnostic (IVD) test kits, glucose monitors, and ultrasound probes—rely on precision components to deliver accurate, consistent results. Precision mold parts ensure these devices perform flawlessly:
Guide Pins for IVD Test Strip Molds: IVD test strips require precise alignment of reagent layers to ensure accurate detection of biomarkers (e.g., COVID-19 antigens, blood glucose). Our precision guide pins (with a tolerance of ±0.0003mm) ensure the mold’s upper and lower halves align perfectly, preventing reagent layer misalignment that could lead to false-positive or false-negative results. A global IVD manufacturer reported a 90% reduction in test strip inaccuracies after switching to our guide pins.
Cooling Inserts for Ultrasound Probe Casings: Ultrasound probes generate heat during use, so their casings must be made from heat-resistant, biocompatible plastics (e.g., PC/ABS). Our precision cooling inserts feature micro-channels (as small as 0.5mm) that 均匀 distribute coolant, ensuring the probe casing cools evenly and maintains dimensional stability—critical for preserving the probe’s acoustic performance.
3. Disposable Medical Devices: Sterility & Consistency for Mass Production
Disposable devices—such as syringes, catheters, and surgical gloves—are used once and discarded, but their quality is still vital to preventing infections and ensuring clinical safety. Precision mold parts enable their high-volume, sterile production:
Valve Gates for Syringe Barrel Molding: Syringe barrels require a smooth, uniform inner surface to ensure precise fluid delivery. Our precision valve gates (with a response time of 0.005 seconds) control the flow of molten plastic into the mold, eliminating "flash" (excess plastic) and ensuring the barrel’s inner diameter remains consistent (±0.002mm) across millions of units. This reduces the risk of fluid leakage and ensures accurate medication dosing.
Corrosion-Resistant Bushings for Catheter Molds: Catheters are sterilized using ethylene oxide or gamma radiation, which can degrade standard mold parts. Our bushings are made from medical-grade stainless steel (316L) and coated with passivation layers to resist corrosion, maintaining their precision across 1 million+ sterilization cycles. For a leading catheter manufacturer, our bushings extended mold life by 40% and reduced production downtime by 30%.
4. Surgical Instruments: Durability for Repeated Use
Surgical instruments—such as scalpels, forceps, and laparoscopic tools—require sharp edges, precise hinges, and resistance to repeated sterilization. Precision mold parts ensure these instruments meet the demands of the operating room:
Precision Pins for Laparoscopic Tool Hinges: Laparoscopic tools (e.g., graspers, scissors) rely on small, durable hinges to operate inside the body. Our precision hinge pins (machined with grinding technology) have a surface finish of Ra ≤ 0.005μm, ensuring smooth movement and preventing jamming during surgery. These pins withstand 500+ autoclave cycles without wear, extending the instrument’s lifespan by 50%.
Micro-Mold Cavity Inserts for Surgical Blade Handles: Surgical blade handles require ergonomic designs and non-slip textures to ensure surgeon control. Our micro-mold cavity inserts (etched with laser technology) create nano-level textures on the handle surface, improving grip while maintaining biocompatibility. A leading surgical instrument brand reported a 60% reduction in handle-related surgical errors after adopting our inserts.
Our Medical-Grade Precision Mold Parts: Built for Compliance & Safety
What sets our precision mold parts apart in the medical device industry? It’s our unwavering focus on meeting the sector’s unique needs—from biocompatibility to regulatory traceability:
1. Medical-Grade Materials & Coatings
We use only FDA-approved, biocompatible materials for our mold parts, including:
316L Stainless Steel: For bushings, guide pins, and ejector pins—resistant to corrosion, sterilization, and particle shedding.
PEEK (Polyether Ether Ketone): For non-metallic parts (e.g., cooling inserts)—biocompatible, heat-resistant, and compatible with all sterilization methods.
Ceramic Coatings (Alumina, Zirconia): For high-wear parts (e.g., valve gates)—prevents metal leaching and maintains precision over long cycles.
All materials come with full traceability certificates (including lot numbers, material composition, and test results) to support regulatory compliance.
2. Ultra-Precision Manufacturing Technologies
Our production facilities are equipped with state-of-the-art technology to achieve medical-grade precision:
Wire EDM (Electrical Discharge Machining): Creates micro-scale features with tolerances as tight as ±0.0001mm—ideal for implantable device molds.
Laser Micromachining: Etches nano-level textures on mold parts (e.g., catheter handles) without damaging the material’s biocompatibility.
CMM (Coordinate Measuring Machines) with Micron-Level Sensors: Inspects every part to ensure compliance with dimensional standards, with 100% of parts tested before delivery.
3. Regulatory Compliance & Quality Assurance
We maintain strict adherence to global medical standards, including:
ISO 13485 Certification: Our quality management system is certified to meet medical device manufacturing requirements.
FDA QSR 820 & EU MDR Compliance: We provide detailed documentation (e.g., DHRs, DMRs, material certificates) to support device registration and audits.
Cleanroom Manufacturing: Critical parts (e.g., implant mold inserts) are produced in Class 8 cleanrooms to prevent contamination.
4. Customization for Medical Innovation
We partner with medical device manufacturers early in the design phase to develop custom mold parts for new devices. Whether it’s a micro-mold insert for a next-generation neural implant or a corrosion-resistant valve gate for a disposable diagnostic tool, our engineering team uses CAD/CAM software and finite element analysis (FEA) to optimize part design—reducing development time by 35% and ensuring first-time mold success.
Partner with the Leader in Medical-Grade Precision Mold Parts
In the medical device industry, where a single defect can put patient lives at risk, your choice of precision mold parts supplier is critical. We have over 15 years of experience supplying medical-grade precision mold parts to global device manufacturers, with a track record of delivering solutions that meet the most demanding requirements—from implantables to diagnostics.
Our commitment to medical excellence includes:
100% biocompatible, traceable materials
Compliance with ISO 13485, FDA QSR 820, and EU MDR
Customized solutions for micro-devices, implantables, and disposables
Fast lead times (2-3 weeks for prototypes, 4-6 weeks for mass production)
24/7 technical support for mold maintenance and troubleshooting
Whether you’re developing a life-saving implant, a high-precision diagnostic tool, or a sterile disposable device, our precision mold parts are designed to help you meet regulatory standards, ensure patient safety, and accelerate time-to-market. Let’s partner to build the next generation of reliable medical devices—contact us today to discuss your project needs.
Related Posts
- Precision Irrigation mold cavity and spare parts
- zefu-mold
- 1053